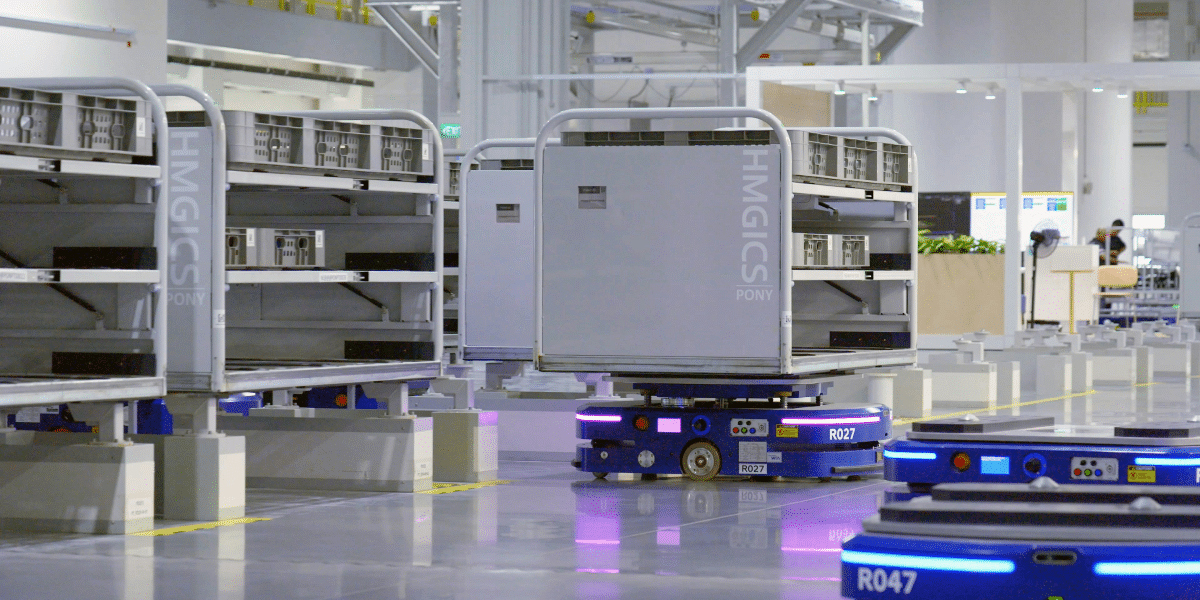
Increasingly, facilities are seeing autonomous mobile robots (AMRs) support tasks like sample transportation, supply delivery, and hazardous waste disposal. AMR robots’ ability to navigate independently relieves skilled professionals from mundane tasks and improves workflow efficiency, but only if they can meet exacting standards. The sensitive nature of Life Sciences and Healthcare facilities requires stringent regulatory compliance to ensure safety, reliability, and data integrity at all times.
A mobile robot often shares spaces with researchers, patients, and staff in labs, hospitals, and other healthcare settings. Compliance mandates that these autonomous robots interact safely with humans, using reliable sensors and software algorithms to avoid collisions and potential harm. Standards like ISO 36006:2020 and ANSI/RIA R15.08 provide specific guidelines for manufacturers to engineer safety features, making AMRs safe partners in dynamic environments.
Beyond safety, reliability is a cornerstone of compliance in regulated industries. Mobile robotics must perform consistently and predictably, especially when handling sensitive or critical materials. This means consistent performance in AMR navigation, task execution, and data logging. This level of reliability reduces human error, minimizes downtime, and ensures operational efficiency within demanding research and healthcare environments.
Why Compliance is Essential in Navigation Robotics
Safety: Mitigating Risks within Robot Navigation Methods
Standards like the ISO 36006:2020 and the ANSI/RIA R15.08 help mitigate the risks of unexpected AMR and human/obstacle encounters.
- Risk Assessment: Compliance standards mandate a thorough risk assessment process, identifying potential hazards in the autonomous robot’s operating environment.
- Collision Avoidance: Autonomous mobile robots must have reliable sensor systems (LiDAR and ToF sensors and additional cameras and sensors) to detect and avoid obstacles and prevent accidents.
- Speed Control: Speed limitations, especially in shared human-robot workspaces, are essential to minimize the impact of potential collisions. AMR robots must be equipped to regulate their speed dynamically based on traffic and potential hazards.
- Emergency Stop (E-stop) Functionality: Easily accessible e-stops are mandated for quick intervention in case of unexpected events. Navigation-based mobile robots must be prepared to mitigate disruption risk along their calculated paths.
- Human-Robot Interaction (HRI): Standards address safe collaboration zones between humans and mobile robots, including visual indicators, audible alerts, and clear communication of the robot’s intended path.
Reliability: Consistency of the Robot Navigation System
Another critical aspect of regulatory compliance is the ability of an AMR to perform tasks consistently and accurately without the natural variability of a human worker. Any deviation in any robot type’s behavior can lead to costly consequences, such as contaminated samples, the delivery of incorrect materials, or even delays in time-critical patient treatments.
- Process Validation: AMRs performing tasks within regulated industries must demonstrate reliable and repeatable behavior to be considered compliant and ensure consistent quality and safety.
- Predictability: Consistent performance allows for accurate planning and scheduling in production environments. Operators can rely on the AMR to complete tasks within defined timeframes.
- Error and Downtime Minimization: Repeatable behavior reduces the risk of errors occurring during operation, ensuring the integrity of products and processes. It also minimizes downtime associated with malfunctions or errors.
Data Integrity: Accuracy and Traceability in All Robot Types
Data generated by autonomous robots has direct implications for research outcomes, patient care, or regulatory audits. A compliant mobile robot will have robust data logging mechanisms with timestamping, user tracking, and protection against unauthorized modification.
- Accurate Data Logging: Compliance with regulations like CFR 21 Part 11 ensures that AMRs have robust data logging mechanisms with timestamping for accurate record-keeping, traceability, and audit preparedness.
- User Tracking: Traceability is maintained through user tracking functionalities within the AMR system.
- Data Protection: Protection against unauthorized data modification safeguards the integrity of information collected by the AMR.
Navigating the Navigation Robots Regulatory Landscape
With each new robotic advancement, standards and regulations are being updated and refined to keep ahead of potential risks. This is especially critical given the rapid pace of recent evolution in AMR robot types. AMR manufacturers and users must ensure their robots comply with evolving regulations and relevant standards. This fosters a safe and reliable operating environment and opens doors for AMRs to be integrated into previously restricted sectors.
- ISO 36006:2020: Safety requirements for collaborative industrial robot systems. While focused on collaborative robots, sections of this standard offer insight into safety measures for AMRs working in proximity to humans.
- ISO 3691-4:2020: While this standard focuses on larger industrial trucks and their systems, some general safety principles may still apply to smaller, mobile lab-oriented AMRs. It’s essential to consult with regulatory experts to determine the level of applicability of this standard to specific AMR implementations.
- ANSI/RIA R15.08 – Part 1–2020: A more recent standard explicitly addressing the safety of mobile robots like AMRs.
Sector-Specific Regulations for Mobile Robotics
- FDA Regulations: The Food and Drug Administration (FDA) regulates medical devices. Navigation robots used in healthcare may need to comply with FDA design, validation, and documentation guidelines.
- GxP Guidelines: Good laboratory practice (GLP), good clinical practice (GCP), and good manufacturing practice (GMP) guidelines ensure quality and integrity in their respective domains. AMRs operating in these environments must facilitate compliance with GxP requirements, particularly data handling, audit trails, and workflow validation.
Case Study – Quasi Robotics’ Approach to Compliance
Quasi Robotics brings reliable and regulation-compliant AMR robot automation to everyday laboratory processes. Both Model R2 and Model C2 robots incorporate features that align with the latest requirements, while CFR 21 Part 11-compliant software ensures comprehensive audit readiness.
Model C2 and Model R2: Enabling Compliant Research Workflows
Models C2 and R2 are tailored solutions for clinical and regulated research environments. Here’s how incorporated features meet the challenges of compliance while streamlining operations:
- Precise Navigation and Repeatable Workflows: An advanced sensor array coupled with a robot navigation optical flow system enables reliable movement that minimizes potential disruptions to experiments or care processes
- Integrated Safety Systems: LiDAR and ToF-based obstacle detection and emergency stop functionality adhere to standards and reduce operational risk in shared lab and facility workspaces.
- Traceable and Detailed Data Logging: Timestamped logging of operational data and user actions facilitates GxP compliance for auditability and research integrity.
- Customizable Grippers for Delicate Handling: For Model R2, customizable end grippers with specialized sensors offer precise and delicate manipulation of sensitive materials like biological samples and fragile medical equipment.
CFR 21 Part 11 Compliant Software
Quasi’s commitment to regulatory cooperation extends to the software controlling the AMR. Full CFR 21 Part 11 compliance means the system provides comprehensive audit trails, meticulously documenting every interaction and modification. This robust data logging is essential for research reproducibility, regulatory audits, and demonstrating a thorough quality system in healthcare settings. It provides the necessary traceability supporting GxP guidelines.
Key Takeaway
By carefully considering navigation control strategies, safety protocols, and robust data handling, AMRs transition from innovative tools to trusted partners in laboratories, hospitals, and research facilities
Compliance navigation robots can significantly reduce errors, streamline workflows, and improve overall research or patient care outcomes by carefully attending to safety, reliability, and data integrity. Quasi Robotics is committed to enabling this transformation by designing AMR solutions with compliance at their core. Through features like sophisticated mobile robot navigation, customizable sensor-based end effectors, and meticulous data logging, Quasi robots address the stringent demands of regulated environments.
As AMR technology evolves, so will compliance standards and regulations. The future of AMRs in these sensitive industries lies in a future-proof approach grounded in upholding compliance. This will unlock the potential for AMRs to automate tasks and generate reliable data that can drive new scientific discoveries and advances in healthcare. This allows the widespread adoption of AMR robots that seamlessly integrate into heavily regulated environments.
Published By: Aize Perez